Calypso Knee System Availability
Calypso knee system availability. The Calypso Knee System is an extra-capsular knee implant designed to fill the therapeutic gap between conservative care and more invasive surgical interventions for knee osteoarthritis. An artificial joint made of metal plastic or. Is a leading provider of cloud-enabled cross-asset front-to-back solutions for financial markets with over 40000 users in 60 countries.
CAUTION Investigational Device. The Calypso System is an implantable unicompartmental joint unloader for patients with medial knee osteoarthritis. Researchers are studying the Calypso Knee Device developed by the company Moximed in 80 patients before it would be available nationwide.
The first generic version of the EpiPen and EpiPen Jr auto-injector was approved by the FDA in August 2018. This generic auto-injector is available in 03mg and 015mg strengths and is marketed by Teva. Body Mass Index BMI of 35 Weight 300 lbs 3.
It may help prevent or delay total knee replacement according to the Fremont Calif. Experts suspect that if the trial is successful patients may be able to access the Calypso Knee System shortly after the trial ends in 2025. Flanigan expects the Calypso Knee System will soon be available to patients across the country.
80 subjects will be enrolled in this study at up to 10 investigational sites located in the US. Following a minimally invasive total robotic knee replacement about 95 percent of patients are able to leave the hospital within one day of surgery while some are able to leave the same day. The uni-compartmental knee is designed to resurface one-third or one compartment of the knee when only one compartment is diseased.
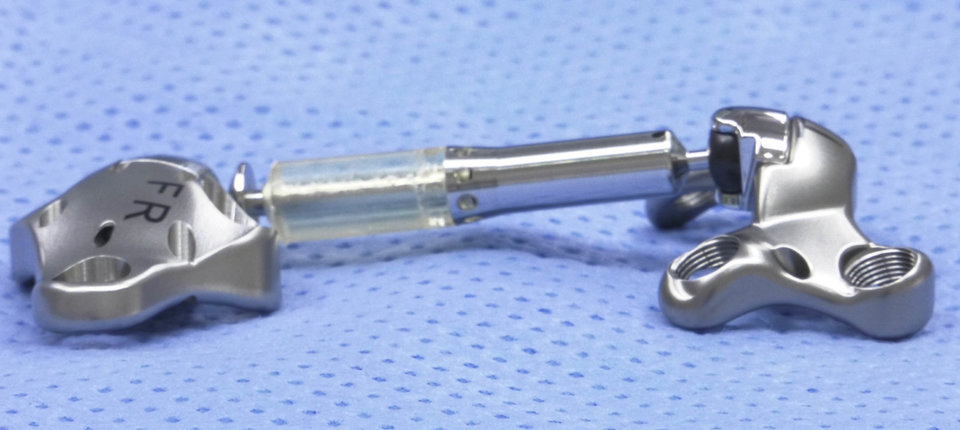
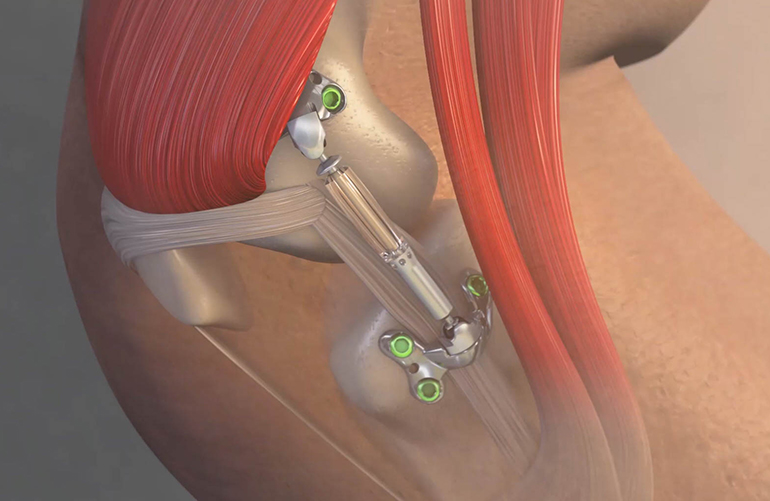
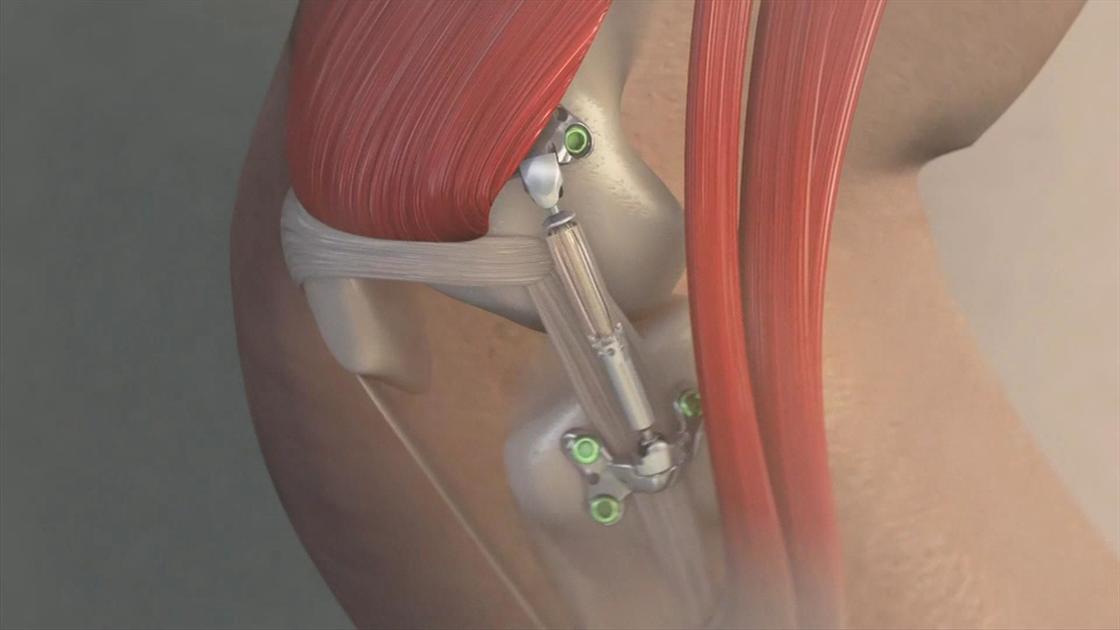
The Calypso Knee System developed by Moximed Inc is designed to act as a shock absorber for the inner knee. The UKU is implanted under the skin alongside your knee joint. Calypso Technology Inc.
The Calypso is designed to work like a shock absorber for the knee by restoring a more normal load balance within the joint. Participants age - 25 to 65 years 2.
Calypso Technology Inc.
The UKU is implanted under the skin alongside your knee joint. The unicompartmental knee unloader UKU is an implant that works like a shock absorber for your knee. The advantages of the outpatient procedure include. Known as the Calypso Knee System the device was developed by Hayward California-based Moximed Inc. The first generic version of the EpiPen and EpiPen Jr auto-injector was approved by the FDA in August 2018. An artificial joint made of metal plastic or. The Calypso Knee System developed by Moximed Inc is designed to act as a shock absorber for the inner knee. You generally have a two- to four-week recovery compared to the two-to-three-months needed to recover from traditional knee surgery. Currently the Calypso Knee System is unavailable to the public but looks like a promising alternative to knee replacement surgeries in the future.
Researchers are studying the Calypso Knee Device developed by the company Moximed in 80 patients before it would be available nationwide. You generally have a two- to four-week recovery compared to the two-to-three-months needed to recover from traditional knee surgery. According to the company the CalypsoKneeSystem treats OA in the inner knee and is designed to provide support outside of the knee joint without altering the anatomy or removing any tissue from the knee itself. It may help prevent or delay total knee replacement according to the Fremont Calif. Every year a number that continues to grow. An artificial joint made of metal plastic or. Knee osteoarthritis pain on the inner side of knee that has continued after at least 6 months of non-operative treatment.



/GettyImages-1226109671-8738d91de6514dd6b08cb2d6c0740c2f.jpg)


/cloudfront-us-east-1.images.arcpublishing.com/gray/X2M24DLMRJOYXGTU6L6JNEN4OU.jpg)








:max_bytes(150000):strip_icc()/doctor-discussing-outcomes-with-a-patient-1159700291-76f2e1ce71794d90bce47179f5274d56.jpg)
:max_bytes(150000):strip_icc()/kneetreat-bac06dd0affd4b928d34c12e181da018.jpg)



/knee_replacement-d94685eb137b454484c8e819c61b4a51.jpg)

















Posting Komentar untuk "Calypso Knee System Availability"