Can A Closed System At Equilibrium Do Work
Can a closed system at equilibrium do work. Based on kinetic theory heat is defined as the energy associated with the random. Nothing can be added to the system or taken away from it apart from energy. A cell in our body is not in equilibrium.
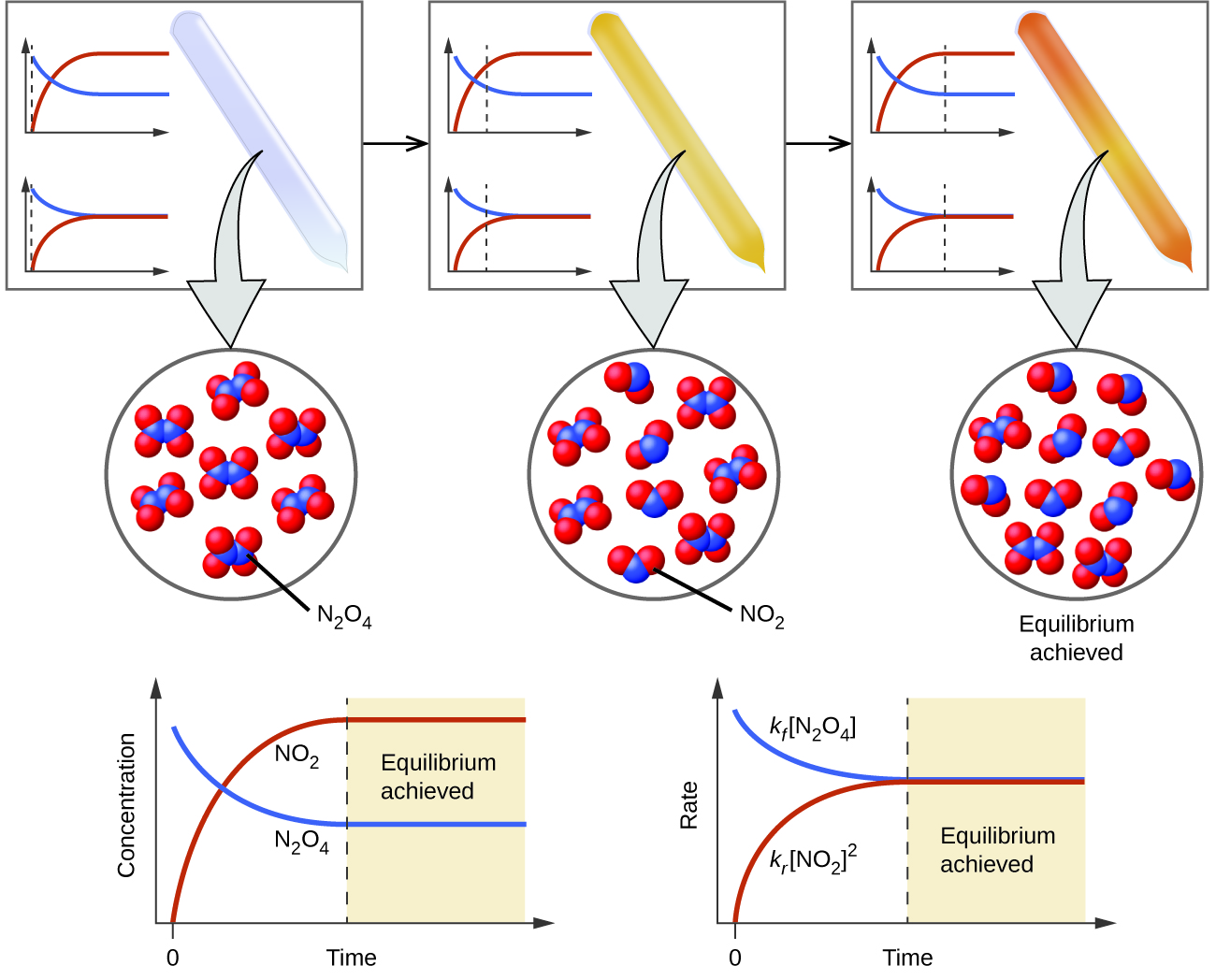
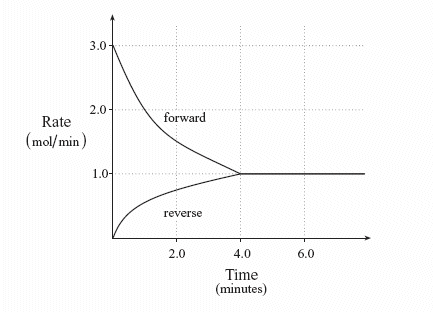
Firstly lets clarify what equilibrium is. N 2O 4g 2NO 2g ΔH 5720 kJ. Reversible reactions in closed systems reach equilibrium where the rates of forward and reverse reactions are constant.
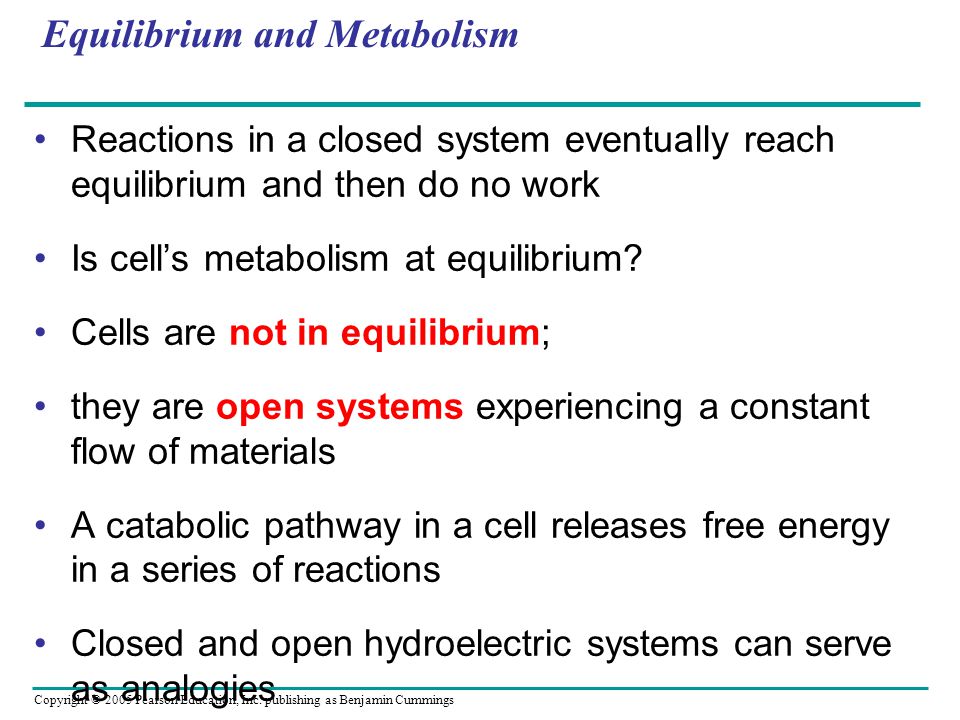
Temperature is the same everywhere. Therefore the nal state will also be di erent. The constant flow of materials in and out of the cell keeps the metabolic pathways from ever reaching equilibrium and the cell continues to do work throughout its life.
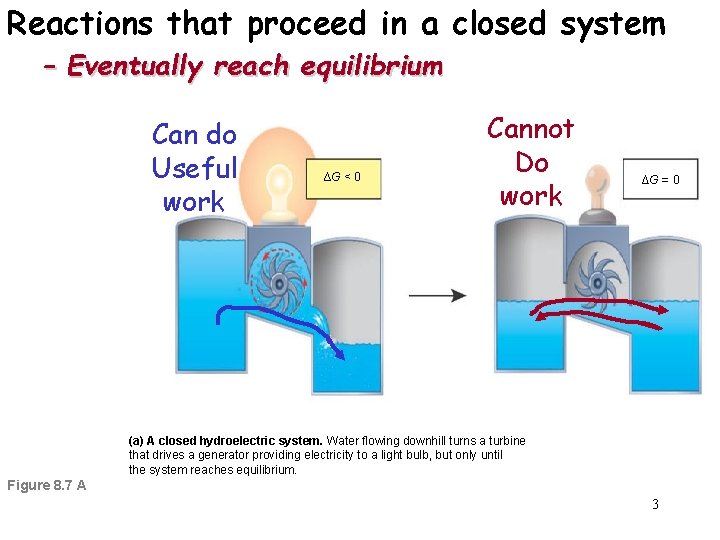
So you cant do work. Free energy is at a minimum. A closed system can be used when conducting chemical experiments where temperature is not a factor ie.
In addition if the variation of that potential is positive work is done on the system. That happen in a closed system eventually reach equilibrium. In a closed system it is possible for reactions to be reversible such as in the demonstration above.
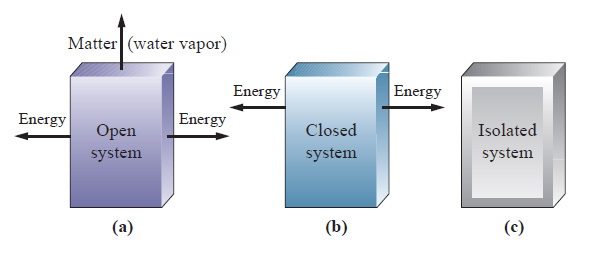
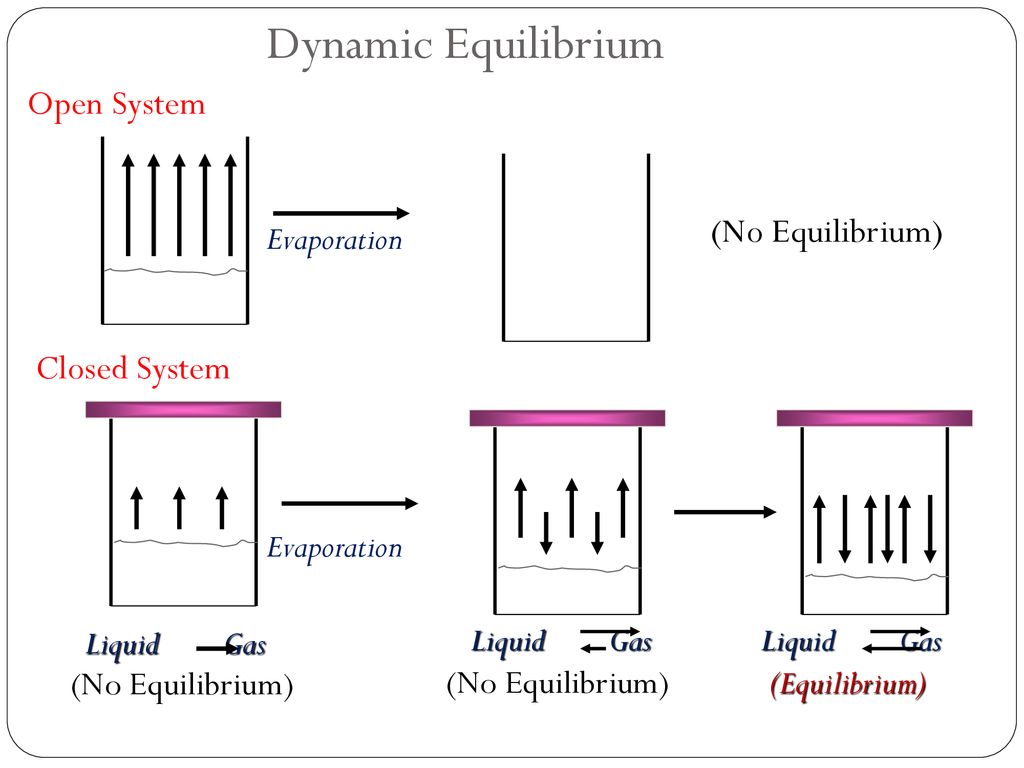
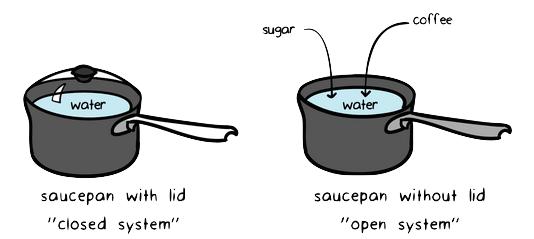
A closed system can exchange energy with its surroundings through heat and work transfer. What Is A Closed System In Chemistry. A cell that reaches the metabolic equilibrium is dead.
The positive ΔH value tells us that the reaction is endothermic and could be written. Equilibrium does not happen only in a closed system.
Nothing can be added to the system or taken away from it apart from energy.
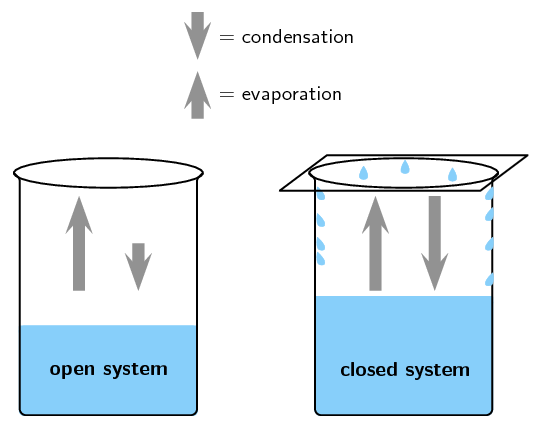
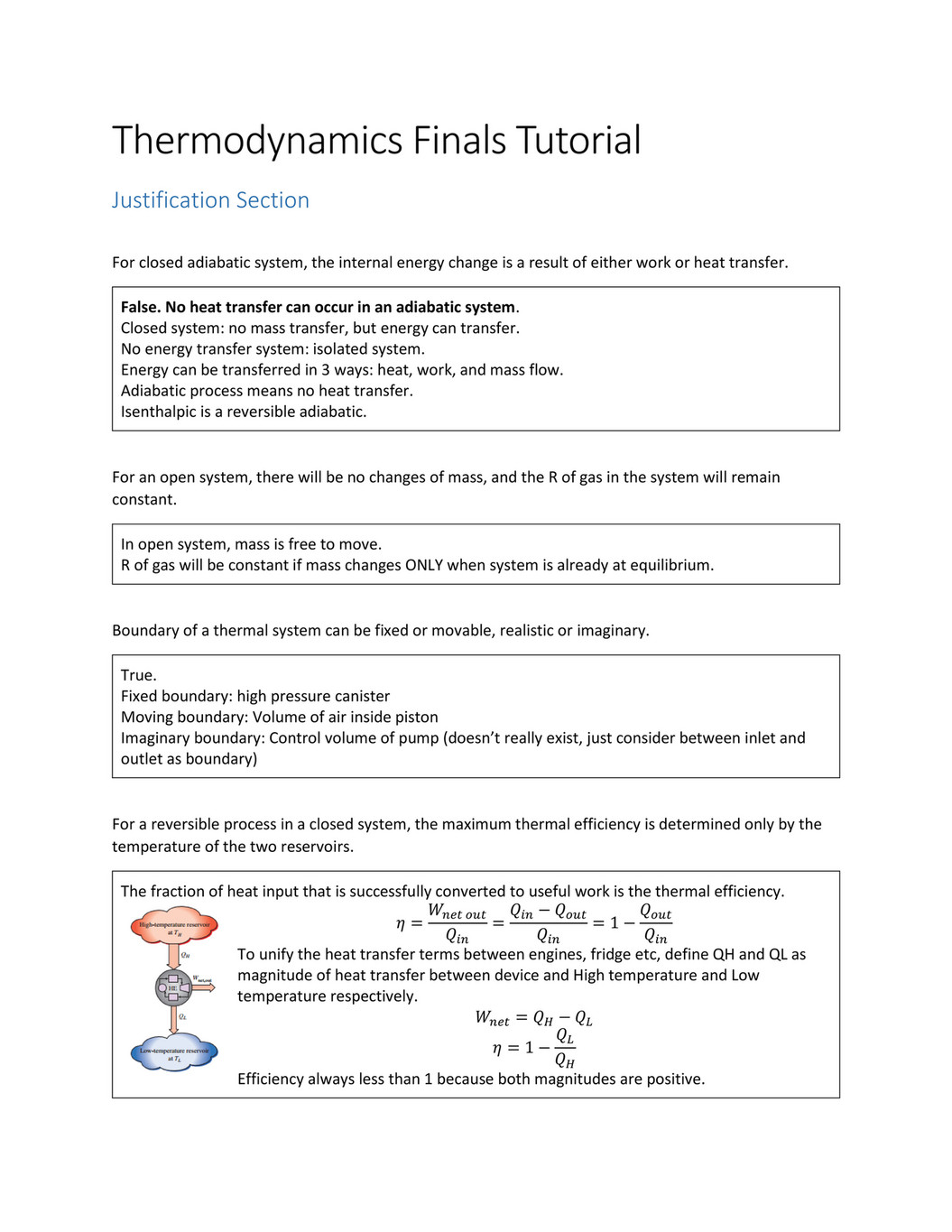
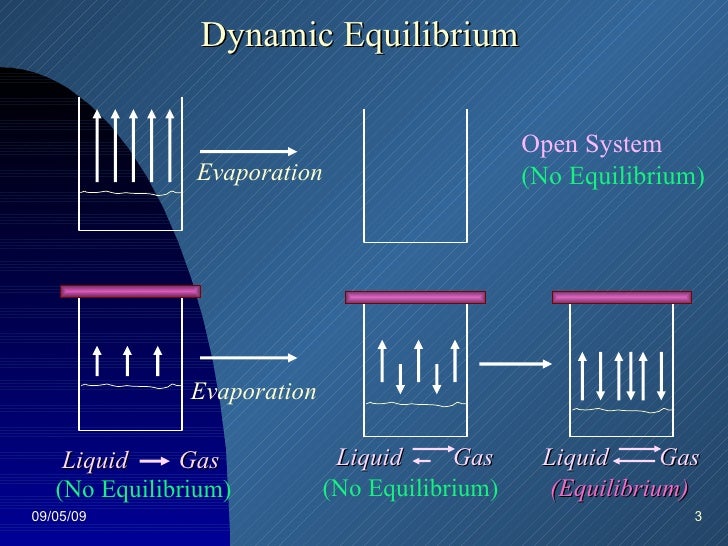
This is not a cheat sheet type of excuse for solving a problem. No systems at equilibrium are at a minimum of G free energy so they can do no work. It happens in open systems also. This is not a cheat sheet type of excuse for solving a problem. Equilibrium does not happen only in a closed system. Nothing can be added to the system or taken away from it apart from energy. Most systems are known as an open system which can exchange energy andor matter with its surroundings Figure. In thermodynamics a closed system can exchange energy as heat or work but not matter with its surroundings. 20 This is called a quasi-equilibrium process.
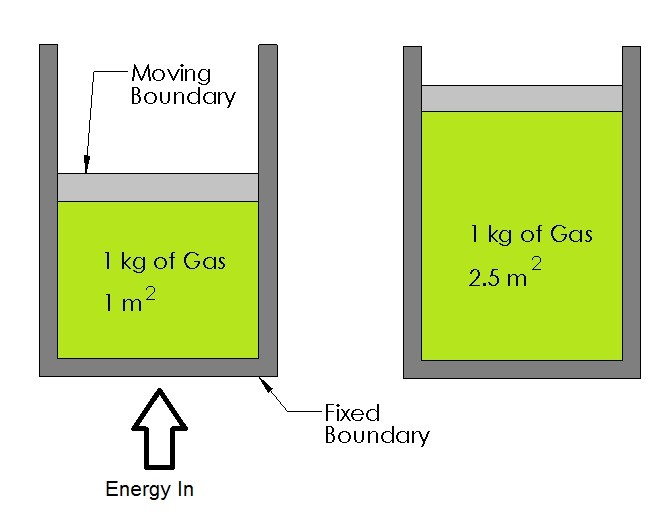
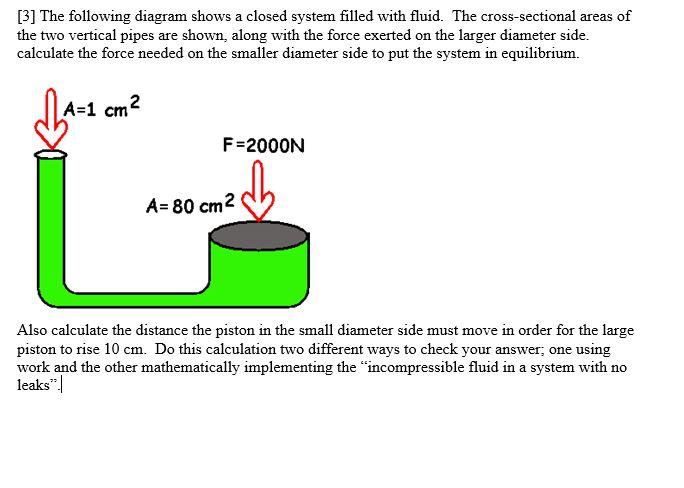
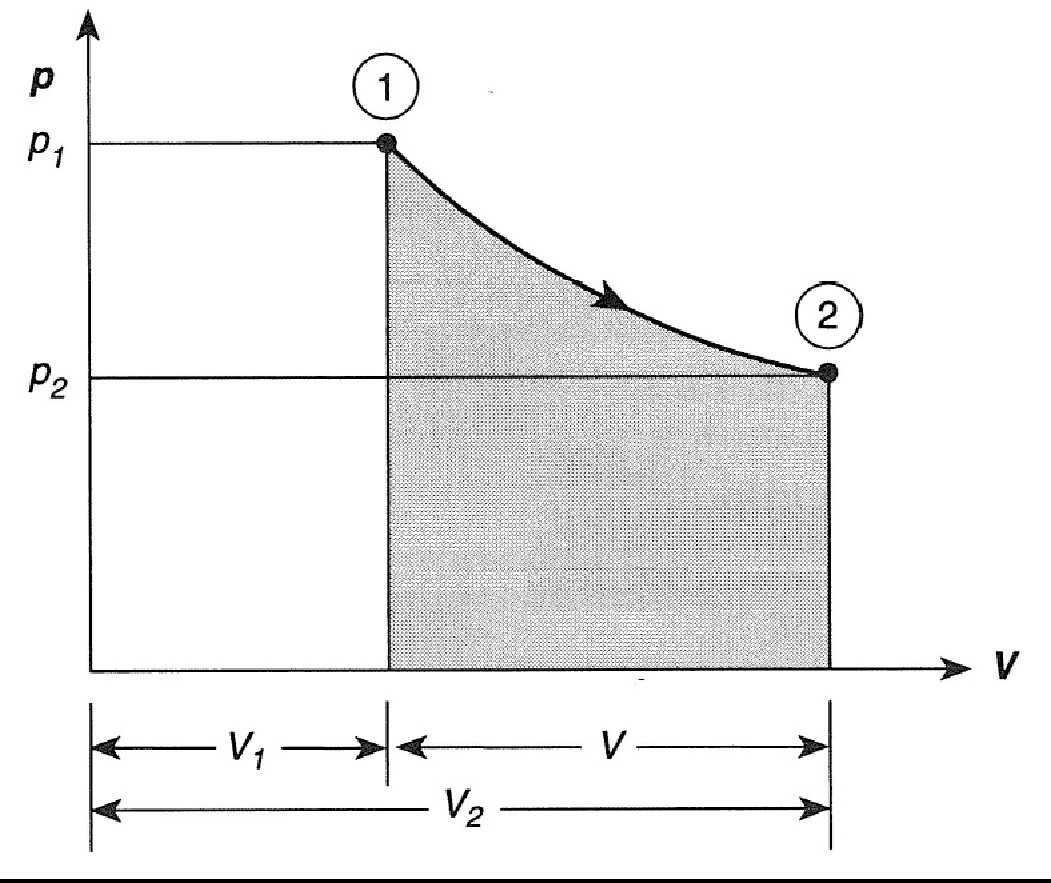
N 2O 4g 2NO 2g ΔH 5720 kJ. The total work done depends on the way leading to the equilibrium. Want this question answered. If a thermally isolated system is in non-equilibrium state it may do work on some external bodies while equilibrium is being established. In a closed system it is possible for reactions to be reversible such as in the demonstration above. Equilibrium can be a tricky concept to understand but this graphic tries to make it a little clearer. In addition if the variation of that potential is positive work is done on the system.





















Posting Komentar untuk "Can A Closed System At Equilibrium Do Work"